KERATOS HH
TECHNICAL DATA
Product: Keratos HH
INCI: Hydrolyzed Keratin
CAS: 69430-36-0
Preservative: Phenoxyethanol + Caprylyl glycol
Free of parabens and formaldehydeProduct dermatologically tested
External use only added to cosmetic products with recommended dosages.
Manufacturer: Elberbio Research and Development Ltd.
the Keratos HH
DESCRIPTION
Keratin is an insoluble fibrous protein and one of the most important and abundant in nature, constituting almost the totality of our epidermis, hair and nails. Like collagen, it has a structural function. It consists of about 18 amino acids, the main one being cysteine. Keratos HH was specially developed, seeking to obtain a protein hydrolysate consisting almost exclusively of amino acids and dipeptides. Due to their small molecular sizes and relative hydrophobicity, these compounds have a high penetrating power in hair, skin and nails.
PROPERTIES
Keratin and its hydrolysates are added in cosmetic formulations in order to give desirable characteristics, such as substantivity to the hair, hydration and elasticity to the skin and strengthening of the nails.
APPLICATIONS
Keratos HH is especially indicated as active in cosmetic formulations, such as shampoo, sprays for hair restructuring, moisturizing creams for the skin and strengthening nail.
SUGGESTED DOSAGES
Table 1 suggests dosages for Keratos HH in cosmetic formulations. However, different dosages can be used depending on the desired result and the time in which it is desired to achieve it.
Table 1. Cosmetic products and suggested dosages of Keratos HH.
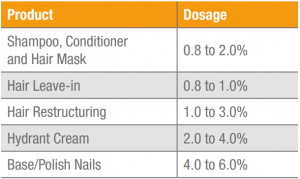
PRESENTATION
White opaque plastic packaging – 5L, 12.5L and 20L – (other volumes when requested).
STORAGE
Keratos HH should be kept in a closed container, away from heat, in a cool and ventilated place. Discreet precipitation may occur due to the association between peptides, not resulting in loss of product quality.
BEST BEFORE
24 months from the date of manufacture.
PHYSICAL-CHEMICAL AND MICROBIOLOGICAL ANALYSIS
The results for the physical-chemical and microbiological analysis of Keratos HH are shown in Tables 2 and 3. These results may vary under other conditions and the analytical and sensorial methods employed.
Table 2. Results for physical-chemical and sensorial analysis of Keratos HH, lot HEQL529119.
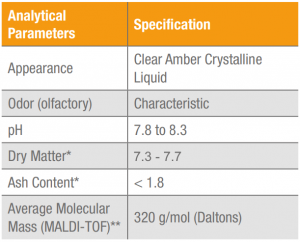
(*) ADOLFO LUTZ INSTITUTE. Analytical Norms of the Adolfo Lutz Institute. v.1: Chemical and physical methods 4th ed. Brasilia: Publisher MS, 2005. 1018 p.
(**) Bruker Guide to MALDI Sample Preparation – Instructions for Use. 2015. Bruker Daltonik GmbH. Bremen, Germany.GmbH. Bremen, Germany.
Table 3. Results for microbiological analysis of Keratos HH, lot 5 HEQL529119.
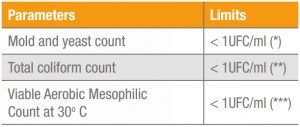
(*) ISO 21527-1: 2008. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of yeasts and moulds.
(**) ISO 4832: 2006. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coliforms.
(***) ISO 4833: 2013. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of microorganisms.microorganisms.
